Antibiotics on the Farm
Supporting animal health and safe food
Antibiotics on the Farm - Pork Checkoff
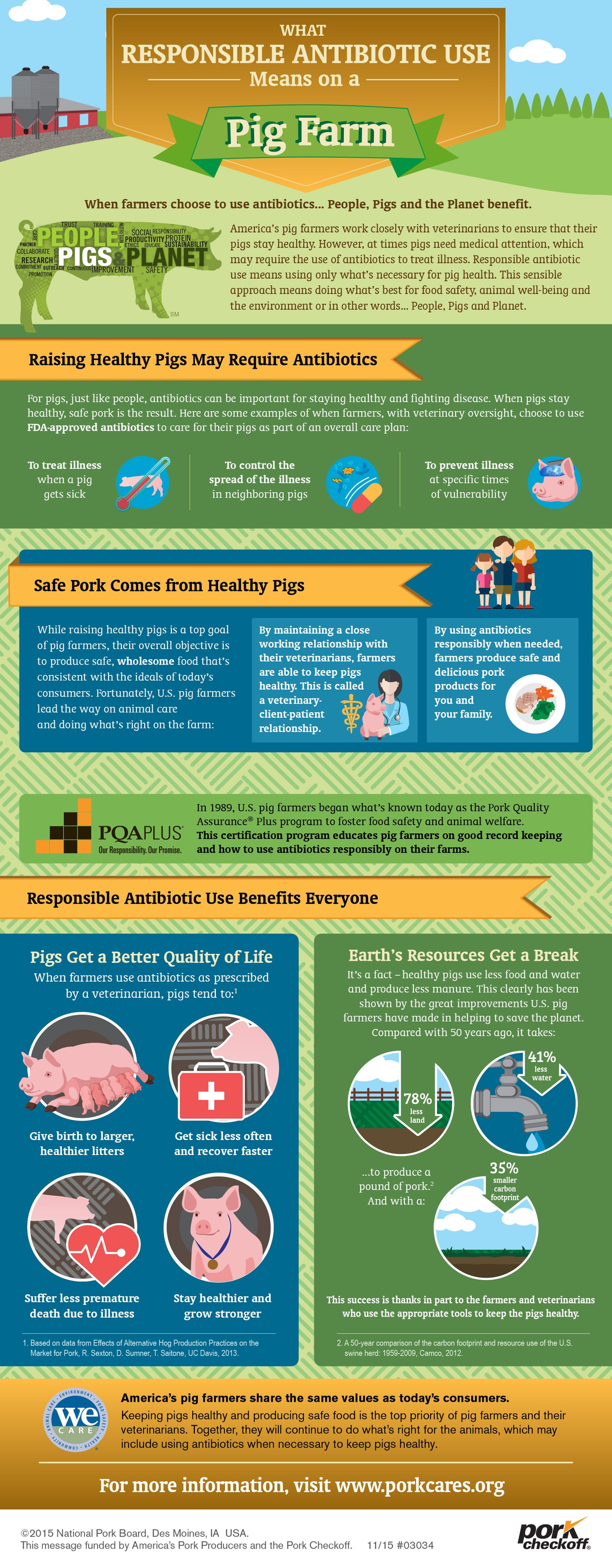
With growing global concerns about antibiotic resistance, people may ask how antibiotic use in animals could affect their health and the health of their families. While many understand that antibiotics used as medication are needed to protect animal health and produce safe food, it’s not always an easy topic to understand or discuss. Some people today ask what farmers are doing to ensure antibiotics are used responsibly to minimize the selection for antibiotic-resistant bacteria and to protect these valuable medications for both human and animal health.
The U.S. pork industry has been engaged in the antibiotic conversation for many years. Today’s pig farmers are committed to helping consumers understand how and why antibiotics are used to keep our food safe and animals healthy through continuous improvement of best practices on the farm, including working closely with their veterinarians. By being open, collaborative and taking a science-based approach, antibiotic stewardship can be improved along with the health of people, pigs and the planet.
Antibiotics are an essential tool for ensuring healthy livestock and safe food
Antibiotics are critical to treat, control, and prevent disease – in humans and animals. Without the responsible and timely use of antibiotics, sickness can spread rapidly on a farm, endangering the health and welfare of animals and the safety of our food.
Our view is simple: Produce healthy livestock, produce safe food.
With a focus on continuous improvement, real change is occurring on farms across the United States. The pork industry is committed to ensuring responsible antibiotic use in animals to protect the efficacy of antibiotics for humans and animals. The industry tests and implements alternative ways to keep pork safe and healthy. Antibiotics are just one of the many approaches in a comprehensive strategy to keep animals healthy and produce safe food. The National Pork Board has adopted a three-point antibiotic stewardship plan that is proactive, collaborative and aggressive in its strategy and scope. Using education, research and communication tactics, the plan will ultimately work for the betterment of people, pigs and the planet.
The U.S. Food and Drug Administration (FDA) approves the use of medically important antibiotics in pigs for treatment, control and prevention of disease. New regulations – FDA Guidance 209 and 213 – were enacted January 1, 2017.
Mitigating disease
Antibiotics are a tool used to uphold Good Production Practices (GPP) to raise healthy farm animals. They are used to prevent, control or treat illness in pigs. Animals on farms, just like people in homes, get sick and without antibiotic treatment many of these animals would suffer needlessly.
In some instances, outbreaks of treatable animal health issues are detected or expected in certain groups of animals or at certain times in their lives. In these cases, use of drugs approved for prevention or control of the disease can dramatically reduce the extent of the disease outbreak. Preventing or controlling the spread of disease is critical to keeping animals safe and healthy—and to prevent suffering or unsafe conditions.
Other disease mitigation strategies include vaccination as well as maintaining good biosecurity practices and an overall sanitary environment. Administering vaccinations is a necessary and important step in maintaining the health of not only individual animals, but all of the pigs on the farm. Combining the use of vaccines along with strict biosecurity measures is very effective in maintaining pig health.
Using antibiotics to care for sick pigs
America’s pig farmers work closely with veterinarians to ensure that their pigs stay healthy. However, at times pigs need medical attention, which may require the use of antibiotics to treat illness. Responsible antibiotic use means using only what’s necessary for pig health. This sensible approach means doing what’s best for animal well-being, food safety and the environment.
When farmers use antibiotics as prescribed by a veterinarian, pigs tend to give birth to larger, healthier litters, get sick less often and recover faster, and suffer less premature death due to illness.
It is inevitable that in every swine production system, animals may become injured or ill.
Antibiotic principles outlined in PQA Plus
When deciding to use antibiotics for treatment, control, and prevention, the National Pork Board encourages producers to implement the following principles:
Principle 1: Take appropriate steps to decrease the need for the application of antibiotics.
Principle 2: Assess the advantages and disadvantages of all uses of antibiotics.
Principle 3: Use antibiotics only when they will provide measurable benefits.
Principle 4: Fully implement management practices described for responsible use of animal health products into daily operations.
Principle 5: Have a working veterinarian/client/patient relationship and follow the responsible antibiotic use guidelines.
You might also like
Jaynie Norman